According to statistics, the average amount of metal corroded annually in the world is about 10-30% of the volume of metal produced. Corrosion of materials causes great damage and loss in terms of economy as well as human life. In order to prevent metal corrosion, we need to understand the agents that cause corrosion to metal, the surface of the material.
There are 02 common types of metal corrosion agents today, we need to pay attention:
Chemical corrosion:
– is a redox process in which the electrons of a metal are transferred directly to substances in the environment.
– Chemical corrosion often occurs in metal parts of machinery or equipment that are frequently exposed to chemicals, oxygen, and steam at high temperatures. The higher the temperature, the faster the metal corrodes.
Electrochemical corrosion
– is an oxidation-reduction process in which the metal is corroded by the action of the electrolyte solution and creates a flow of electrons from the cathode to the anode.
– Electrochemical corrosion usually occurs when the metal pair (or alloy) is left out in moist air, or immersed in an acid solution, salt solution, in pure mineral water, etc.
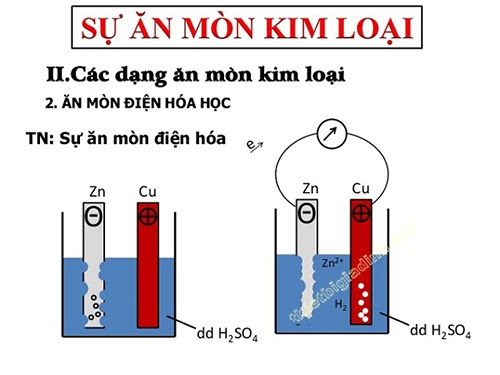
Mechanism of electrochemical corrosion
Cast iron or steel are Fe – C alloys, in which the cathode is Fe crystals, the anode is C crystals. These electrodes are in direct contact with each other and with an electrolyte solution. Thus, the object is corroded in an electrochemical fashion:
+ At the cathode: Fe atoms are oxidized to. These ions dissolve in an electrolyte solution in which some oxygen is already present, where they are further oxidized to.
+ At the anode: The hydrogen ions of the electrolyte solution move to the anode, where they are reduced to free hydrogen, then exit the electrolyte solution.
The Fe crystals are oxidized from the outside to the inside in turn. After a while, the cast iron (steel) object will corrode.
Conditions for the occurrence of electrochemical corrosion
- The electrodes must be different in nature, be it a pair of 2 different metals or a pair of metal with a nonmetal, etc.
- The electrodes are in contact with an electrolyte solution
Without any of the above three conditions, electrochemical corrosion will not occur
In nature, metal corrosion is complex, and both electrochemical and chemical corrosion can occur simultaneously.
Measures against metal corrosion
Surface protection method
- Use sustainable substances to coat metal surfaces such as paints, greases, plastics, etc.
- Clean, store in a dry place
- Western iron is iron coated with tin, corrugated iron is iron coated with zinc. Iron objects are often plated with Nickel or Chromium
- Use a metal as a “sacrifice” to protect metal materials.

References:
- The best anti-corrosion solution for metal today
- Knowledge of industrial paints
- Cleaning metal surfaces by sandblasting
NANOTECH VIETNAM JOINT STOCK COMPANY
Address: No. 1747 Vo Nguyen Giap, Ward 12, Vung Tau City, Ba Ria – Vung Tau Province, Vietnam
Phone: 0254.3515.786 – 0778.828.879
Email: info@nanotechvietnam.com





