What is an anti-corrosion agent?
Anti-corrosion agent or A corrosion inhibitor is a compound mixed into a liquid or gas to reduce the rate of corrosion of a material, usually a metal or alloy. The effectiveness of a corrosion inhibitor depends on the fluid composition, water content, and flow regime. A general mechanism for inhibiting corrosion involves the formation of a covering layer, usually a passive layer, that prevents the penetration of corrosives into the metal. Permanent treatments such as regular chrome plating
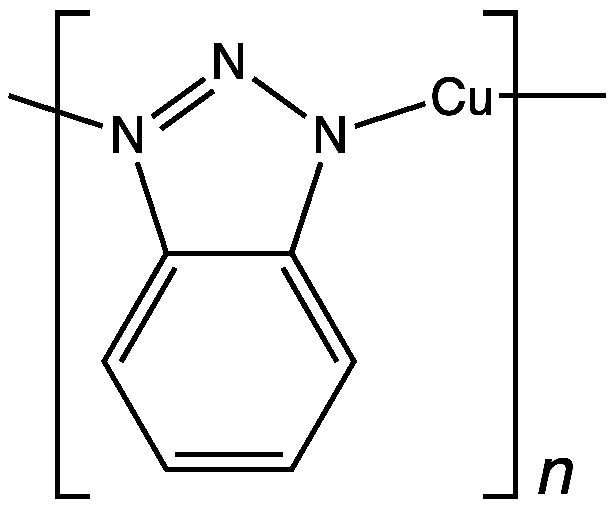
Benzotriazole inhibits copper corrosion by forming an inert polymer layer on the metal surface
Not considered an inhibitor. Instead, corrosion inhibitors are additives added to the liquid surrounding the metal or object that needs protection.
Corrosion inhibitors are common in industry and are also found in commercially available products, often in spray form combined with lubricants and sometimes penetrating oils.
The nature of the corrosion inhibitor depends on the material being protected, usually a metallic object, and on the corrosive agent(s) being neutralized. Corrosive substances are generally oxygen, hydrogen sulfide, and carbon dioxide. Regular oxygen is removed by mitosis inhibitors such as amines and hydrazine: O2 + N2H4 → 2 H2O + N2
In this example, hydrazine converts oxygen, a common corrosive substance, into water, which is generally benign. The inhibitors involved in oxygen corrosion are hexamine, phenylenediamine, and dimethylethanolamine, and their derivatives. Antioxidants such as sulfides and ascorbic acid are also used. Some corrosion inhibitors form a passive coating on the surface by chemical absorption. Benzotriazole is one of the species used to protect copper. For lubrication, zinc dithiophosphates are popular – they leave sulfides on the surface.
The suitability of the corrosion protection of any given chemical for a particular job depends on many factors, including operating temperature.
Some common corrosion inhibitors include:
- Galvanizing: When a thin layer of zinc is plated onto another metal surface (such as iron), it creates a protective layer to prevent water and oxygen contact with the metal surface.
- Anti-corrosion paint: Anti-corrosion paint contains compounds that have the ability to adhere to the surface and create a physical layer to prevent corrosive substances from coming into contact with the surface.
- Stainless metal coating: Uses compounds such as aluminum alloys, stainless steel, and nickel to create a protective layer on metal surfaces.
- Use of anti-corrosion compounds: Many chemical compounds such as chromate, phosphate, or manganese-containing compounds are used to create a protective layer on metal surfaces.
- Apply electrolysis: Electrolysis can be used to create a protective layer on a metal surface by applying voltage to an electrolyte reservoir.
The specific type of corrosion protection will depend on the type of metal or surface to be protected, as well as the environment it will be subjected to.
References:
- S2S PLID TAPE ANTI-CORROR COATING FOR VALVE, FLANGE
- CAUSES AND ANTI-CORROSION SOLUTIONS FOR PIPEES
- APPLICATION OF ANTI-CORROR TAPE – PROTECTING CURRENT MARINE WORKS
- 04 POPULAR TYPES OF ANTI-CORROR TAPE IN INDUSTRY
- THINGS YOU NEED TO KNOW ABOUT COOLING SYSTEM
NANOTECH VIETNAM JOINT STOCK COMPANY
Address: No. 1747 Vo Nguyen Giap, Ward 12, Vung Tau City, Ba Ria – Vung Tau Province, Vietnam
Phone: 0254.3515.786 – 0778.828.879
Email: info@nanotechvietnam.com





